After 35 Years, the EpiPen Gets a Needle-Free Alternative …C0NTINUE READING HERE >>>
Key Takeaways
The FDA recently approved an epinephrine nasal spray called neffy to treat anaphylaxis.The spray is the first needle-free alternative to the EpiPen.Neffy will be available within eight weeks of the FDA approval.
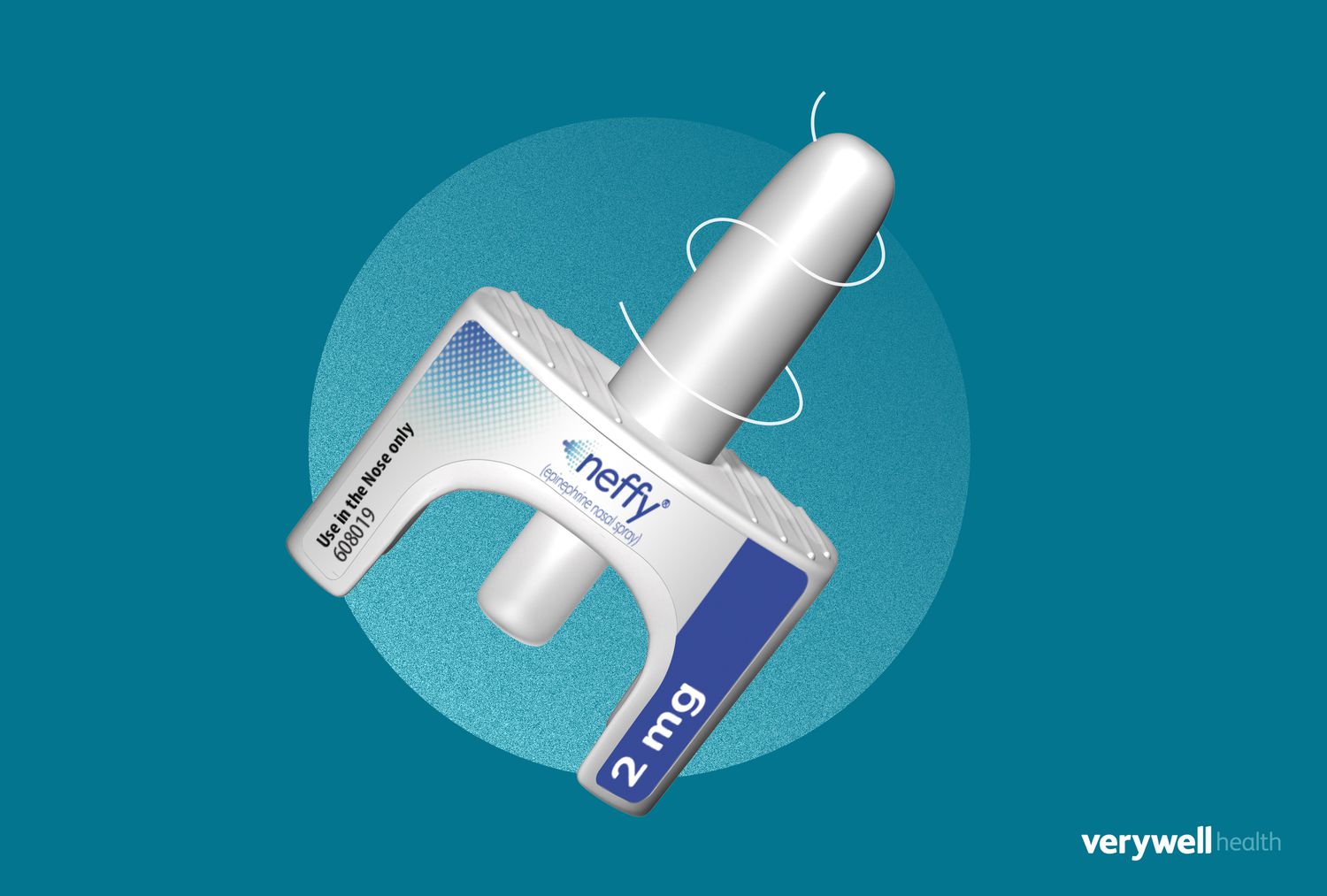
On Friday, the Food and Drug Administration (FDA) approved neffy, a nasal spray alternative to the EpiPen. The device gives adults and children a needle-free option to help stop the onset of anaphylaxis, a life-threatening allergic reaction, making it the first epinephrine emergency product to hit the market in over 35 years.
The approval is the result of five clinical trial studies that tested the effects of neffy against the traditional auto-injector form of epinephrine and found the 2-milligram nasal spray to be just as effective at treating anaphylaxis in adults and children weighing 66 pounds or more.
“I would absolutely start recommending neffy as an alternative to traditional epinephrine autoinjectors,” Alok Patel, MD, a pediatric hospitalist at Stanford Medicine Children’s Health, told Verywell. “The data collected thus far shows that neffy is as effective in managing anaphylaxis, which means it can absolutely be lifesaving.”
What Is neffy and Who Is It For?
Neffy is a nasal spray version of epinephrine designed to quickly help stop an anaphylaxis reaction without the burden of using a needle-based auto-injector. It’s administered similarly to Narcan and Valtoco, emergency medications used to treat opioid overdoses and seizures, respectively.
Neffy is meant to be carried by the millions of children and adults who have been diagnosed with life-threatening allergies to food, insect bites, medications, or other allergens that can lead to anaphylaxis. Currently, 52% of adults with food allergies are prescribed an EpiPen, and 36% believe the auto-injector can cause life-threatening effects. Neffy can be an ideal alternative for those who find carrying an EpiPen too unnecessary or burdensome.
“I hope to see children with food allergies get prescribed neffy to keep at school,” Elizabeth Green, a school nurse in California, told Verywell. “Since it doesn’t have a needle, it will be easier to train staff. Hopefully, it will be cheaper and easier to obtain than the EpiPen.”
While the approval is only for people who weigh 66 pounds or more, ARS Pharma, the manufacturer of neffy, plans to file a supplemental application with the FDA to include children who weigh 33 pounds or more by the end of 2024.
What Is Anaphylaxis?
Anaphylaxis is a whole-body severe immune reaction to an allergen. It often involves life-threatening airway, breathing, and circulation complications that, while rare, can lead to death if untreated. Up to 5% of the U.S. population has experienced anaphylaxis at some point.
The most common causes of anaphylaxis include:
Food allergies (peanuts, tree nuts, eggs, seafood, etc.)
Latex allergies
Insect bites and stings
Medications (antibiotics, aspirin, ibuprofen, etc.)
How to Use neffy
Neffy is a single-use nasal spray. Two doses should be carried at all times if you or a loved one has severe allergies. The drug has a shelf life of 30 months and should be stored at room temperature.
If you’re having a possible anaphylaxis reaction or assisting someone else who is, administer a single dose (2 milligrams) into one nostril. If symptoms do not subside within five minutes, use a second dose of neffy in the same nostril.
Having nasal polyps and or a history of nasal surgery can affect absorption. If this applies to you and you’re seeking neffy, talk to your doctor.
Common side-effects of neffy include:
Increased heart rateNausea or vomitingHeadacheNasal discomfort Stomach painNasal congestion
How to Get neffy
Neffy is available through a prescription. ARS Pharma says it will provide patient support programs to keep costs down, such as:
Medication fulfillment services Financial supportHelp navigating insurance requirements and barriers to accessLimiting 2 doses to just $25 for people with commercial insuranceWill offer a cash price of $199 to those without insurance or high deductibles
Neffy will be available in the U.S. within eight weeks.
What This Means For You
If you or a loved one has severe allergies that can lead to anaphylaxis, talk with your doctor to see if neffy is a good alternative to the EpiPen.
Verywell Health uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy.
ARS Pharma. ARS Pharmaceuticals receives FDA approval of neffy (epinephrine nasal spray), the first and only needle-free treatment for type 1 allergic reactions, including anaphylaxis.
Food and Drug Administration. FDA approves first nasal spray for treatment of anaphylaxis.
American College of Allergy, Asthma, and Immunology. Only 52% of Adults with severe food allergy have been prescribed an epinephrine auto injector.
Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. J Allergy Clin Immunol Pract. 2017;5(5):1169-1178. doi:10.1016/j.jaip.2017.06.031
American College of Allergy, Asthma, and Immunology. Anaphylaxis.
ARS Pharma. Neffy product information.
ARS Pharma. Neffy (epinephrine nasal spray) [drug label].
Thanks for your feedback!
What is your feedback?
Other
Helpful
Report an Error
>